Left atrial appendage closure for stroke prevention
Atrial fibrillation, the most common cardiac arrhythmia in the world, can be responsible for triggering stroke by facilitating thrombus formation inside the left atrial appendage. Left atrial appendage closure for stroke prevention therefore plays an important role in the treatment of patients with atrial fibrillation.
Contact
What is left atrial appendage closure for stroke prevention?
The standard method of treatment for stroke prevention in patients with atrial fibrillation consists of oral anticoagulation therapy. A different treatment option also exists for those patients who do not tolerate this type of therapy due to bleeding complications. This consists of eliminating the source of embolisms by performing a catheter-based procedure that uses a special closure system to close the left atrial appendage (LAA occlusion device). Estimates suggest that 90% of all embolic strokes originate in the left atrial appendage.
The origins of left atrial appendage closure for stroke prevention
The first procedure to implant an occlusion system into the left atrial appendage was performed in 2001. Of the two currently approved occlusion systems available for this procedure, the Heart Center uses the Watchman device. This device consists of a self-expanding flexible nitinol frame structure with fixation anchors and a polyester fabric that covers the atrial-facing surface of the device.
What conditions can be treated with left atrial appendage closure?
Patients with atrial fibrillation and:
- Oral anticoagulation intolerance
- Complications as a result of oral anticoagulation (embolisms or bleeding)
- An unacceptably high risk of bleeding (risk of falls, discontinuation of anticoagulation therapy difficult)
What can left atrial appendage closure achieve?
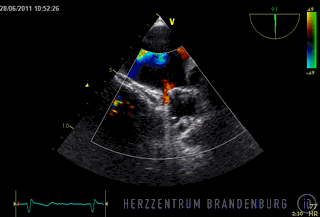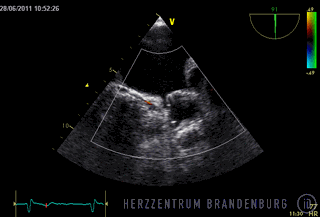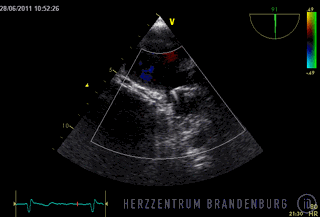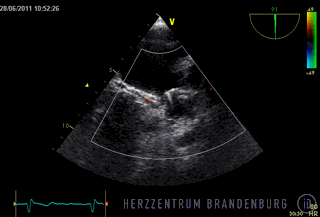
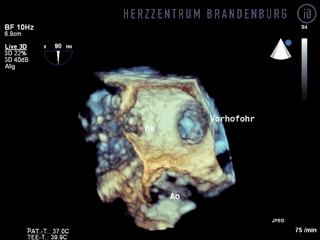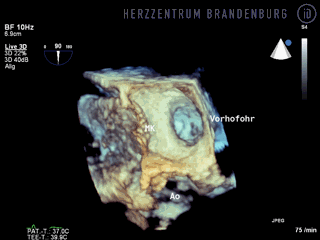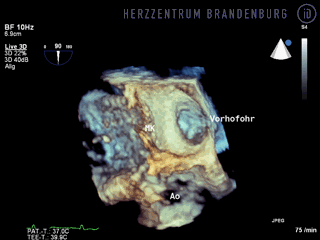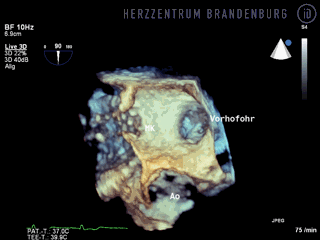
The animation shows a simplified schematic of the LAA occlusion device, which is reminiscent of a plug that is used to permanently close the left atrial appendage (LAA). A long access sheath is introduced via a vein in the groin and advanced to the right atrium. Using echocardiography and x-ray guidance technology, the inter-atrial septum is crossed and the LAA occlusion device is inserted into the left atrium.
Treatment steps involved in left atrial closure for stroke prevention:
- The patient attends an appointment at our hospital. A transesophageal echocardiogram (TEE) is performed in order to establish whether the patient qualifies for LAA closure, and whether the implantation of an LAA occlusion device is in fact feasible and advisable.
- The procedure is performed under general anesthesia in our catheterization laboratory.
- Once a small incision has been made in the right femoral vein, the access sheath is advanced into the right atrium. The inter-atrial septum is crossed and the occlusion device's access sheath is advanced into the left atrium.
- The occlusion device is advanced through the access sheath and deployed in the left atrial appendage. Using x-ray guidance in combination with contrast agents, as well as continuous monitoring using transesophageal echocardiography (TEE), the LAA occlusion device is advanced into the left atrium and positioned in such a way as to completely block the opening to the left atrium.
- The device is released from the introducer system and, over the following few months, it will gradually become covered with endocardium.
Follow-up treatment
Correct positioning of the LAA occlusion device will need to be checked using transesophageal echocardiography at three and six months after the procedure.
Patients will usually require oral anticoagulation therapy for three months following the implantation, and will also need to take acetylsalicylic acid (aspirin) to inhibit platelet aggregation. After the first three months, the majority of patients will need to continue with a combination of acetylsalicylic acid (aspirin) and clopidogrel for a further 3-6 months.